Banana Pest and Disease Management in the Tropical Pacific: A guidebook for banana growers
Chapter VI: IPM Strategies against Black Leaf Streak Disease
Important facts about the biology of Mycosphaerella fijiensis
As mentioned in Section II-3, black Sigatoka or black leaf streak (BLS) disease is a more challenging banana fungal disease than yellow Sigatoka disease. One of the key reasons is that the pathogen of BLS (M. fijiensis) produces more sexual spores (ascospores) than asexual spores (conidia), whereas the reverse is true for yellow Sigatoka disease (M. musicola). Recombination of genetic materials during the sexual reproduction allows for the possibility of selecting progeny that can overcome fungicide toxicity. Table 6-1 summarizes several facts about the biological differences between black and yellow Sigatoka diseases.

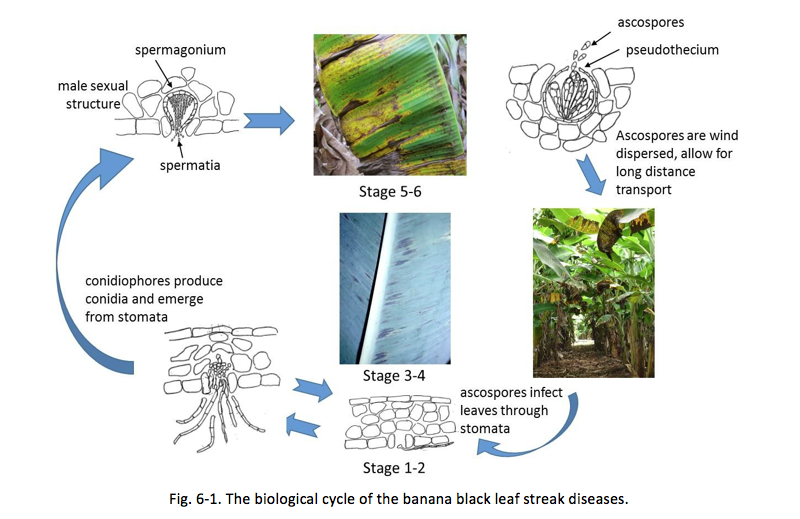
A good understanding of the biology of the black leaf streak disease can help farmers to improve their disease-management strategies against BLS more cost-effectively and sustainably. Ganry et al. (2012) categorized the symptom development of black leaf streak into 6 stages (Fig. 6-1): stage 1 = yellowish depigmentation spots and stage 2 = extension of the lesions to brown streaks on the under-surface of a leaf due to the formation of conidiophores. Stages 1 and 2 are easy to miss with the naked eye. Stage 3 = elongation and enlargement of the lesion visible on the under and upper leaf surface and stage 4 = brown oval lesion; stages 3 and 4 are manifestations of the formation of conidiophores along with spermagonium, the male reproductive structure, on the lower leaf surface and perithecium, the female reproductive structure, on the upper leaf surface. Stage 5 = obvious black lesions on leaf surface and stage 6 = gray necrotic lesions where leaf streaks coalesce and yellow halos for isolated lesion. This is when perithecia mature, are fertilized, and emit ascospores (sexual spores). Ascospores are windborne and can achieve longer-distance dispersal than the water-dispersed conidia. In addition, ascospores go through genetic recombination and thus could develop fungicide-resistant populations. Therefore, a more versatile IPM strategy against BLS should be focused on mitigating the spread of ascospores.
Fouré & Ganry (2008) developed a fungicide-based BLS-management program driven by a forecasting system in Guadeloupe and Martinique. However, this forecast system assumes a limited or nonexistent fungicide-resistant population and relies on not only the availability of several effective systemic fungicides but also the cooperation of area-wide banana growers in managing fungicide resistance. Fouré & Ganry emphasized that fungicide rotation works efficiently if the pathogen pressure is not too high. Some of the practices supplemental to fungicide application include sanitation practices consisting of removal of necrotic and pre-necrotic leaves to eliminate new sources of inoculum; planting resistant or tolerant cultivars; and managing plant nutrition, crop density, and irrigation. This chapter will review some of these practices that are applicable to banana production in Hawai‘i.
Sanitary leaf removal
Sanitary leaf removal means removing BLS-contaminated leaves or leaves showing necrosis or pre-necrotic symptoms. Scientists working with banana research have validated that 5–7 leaves per plant are sufficient to support production of a healthy banana bunch (Ramsey 1990, Daniells et al. 1994, Vargas et al. 2009). Removal of BLS-symptomatic leaves not only is one key practice for removing the inoculum of the disease; it also increases the efficiency and sustainability of chemical control strategies by minimizing the exposure of ascospores to fungicide application. Indirectly, removal of contaminated leaves will also reduce the spray coverage needed, thus reducing the cost and labor of fungicide application. Even if BLS disease is not severe, it is a good IPM practice to routinely maintain only 5–7 leaves per plant to reduce humidity in the crop canopy, which can in turn reduce the infection rate of fungal spores. Guidelines below are “Good sanitary leaf removal” practices for banana growers (Ganry et al. 2012):
- Complete sanitary leaf removal before a fungicide spray, especially if fungicides that are known to trigger fungicide-resistant M. fijiensis populations are used.
- Remove leaves that have reached stages 3, 4, or beyond (see Fig. 6-1).
- In a highly infested field, removal of most of the leaves on the plant might be necessary to revive the plot.
- Cut leaves showing BLS necrosis should be placed upside down, with the upper side against the ground. This is because ascospores are produced on the upper leaf surface (Fig. 6-1). Under very severe conditions, during the first cycle of sanitary leaf removal growers should pile all the cut leaves at the end of a row. This is because only the top leaf in a pile is likely to emit spores, whereas the leaves within the pile are relatively contained.
- Sanitary leaf removal should be practiced weekly, especially in wetter regions where fungus-infection rate is high, and should be treated as the priority control practice.
- This practice should also be accompanied by desuckering to maintain only 3–5 plants per banana mat to further decrease canopy humidity.
- If farmers are seeking to reestablish a severely BLS-infested banana field, rapid conversion to fallow by injecting plants with bananacide (glyphosate) and burying them 2 weeks after the bananacide injection is recommended.
Sanitary leaf removal practices, done correctly and regularly, can reduce more than 80% of spore production of M. fijiensis (Ganry et al. 2012). A banana leaf with BLS that remains hanging on the pseudostem produces spores for 4 to 5 months, while one that is covered on the ground produces spores for 2 to 3 weeks. Area-wide removal of necrotic leaves may be necessary in high-infestation areas for this practice to be effective, so that spores will not be spread from neighboring farms.
BLS-resistant varieties
Planting resistant banana varieties restricts the reproduction of M. fijiensis. However, only few banana varieties are commercially planted in Hawai‘i. Among the two most commonly planted commercial banana varieties in Hawai‘i, ‘Cavendish’ types and ‘Dwarf Brazilian’, locally called “apple banana,” the ‘Dwarf Brazilian’ is more tolerant of BLS disease. However, M. fijiensis can still reproduce on ‘Dwarf Brazilian’. A true BLS-resistant variety is ‘Yangambi Km 5’, an AAA dessert banana. A hypersensitive reaction of the resistant host occurs when the pathogen infects the resistant plant, resulting in blockage of BLS symptoms at an early stage (Fouré et al. 1990). Various diploid banana varieties are used in breeding programs as a source of resistance, such as ‘Paka’ (AA) and some genotypes from the Mlali group, originating from the Comoros archipelago (Beveraggi et al. 1995). Several BLS-resistant hybrids were developed in Honduras by Fundacion Hondureña de Investigacion Agricola (FHIA). In tests conducted in Pohnpei using these hybrids, FHIA-01 (‘Goldfinger’) and FHIA-02 are listed as highly resistant, FHIA-03 (‘Sweetheart’) and FHIA-18 are listed as resistant, and FHIA-23 (progeny of ‘Highgate’, which is a dwarf ‘Bluefields’) is listed as tolerant to BLS (Nelson and Javier 2007, Nelson 2008).
Since M. fijiensis has the ability to overcome selection pressure, a more sustainable approach is to plant partially BLS-resistant banana cultivars that have multiple resistant genes (polygenic) rather than a single resistant gene (monogenic). Fortunately, some of these cultivars have already been introduced into Hawai‘i. These include the banana subgroups ‘Pisang Awak’ (ABB, i.e., ‘Fougamou’) and ‘Mysore’ (AAB).
Crop densities
Growers in Hawai‘i generally plant about 500–700 plants per acre. Planting densities higher than this can create more BLS disease problems. Wider row spacing allows for better airflow and reduced relative humidity within the crop canopy, thus resulting in less infection of the fungus. In Hawai‘i, farmers use either single-row or double-row planting systems. If BLS disease intensity is expected to be high in a region, a single-row planting system might help to avoid severe disease outbreak.
Plant nutrition
Maintaining adequate nutrition of banana plants speeds up their growth so they can outgrow the younger and more susceptible stages quickly. After flowering, banana plants stop producing leaves, they are most vulnerable to BLS. Banana production is generally more productive on the windward side of an island in Hawai‘i; usually banana fields in these areas require 300–650 lbs of nitrogen, 60–120 lbs of phosphorous, and 600–700 lbs of potassium per acre per year. Beside these key macronutrients, banana also requires other macro- and micronutrients (Nelson 2009).
Chemical management of BLS (fungicides)
Although organic farming has gained popularity among new farmers in recent years, effective options for combatting BLS in commercial-scale banana production—which primarily relies on monoculture of banana varieties that are usually not resistant to BLS—are limited. Researchers in Cameroon have experimented with organic fungicides or bio-fungicides such as various essential oils, alimentary additives, organic acids, potassium carbonates, leachates of decomposed banana material (bunch stems, fruits), and bio-control agents. None of these fungicides gave good control of BLSD under high inoculum pressure. However, de Lapeyre de Bellaire et al. (2009) recently demonstrated that the combination of some bio-control agents (Bacillus subtillis and B. pumilis) applied in mixtures with contact fungicides (many of which are OMRI labeled) could allow growers to reduce the amount of fungicide applied.
However, farmers should also not consider conventional fungicides as magic bullets to manage BLS. As explained in the introduction, M. fijiensis has a high capability to evolve due to its preference for sexual reproduction. Depending on the type of fungicides used in a region and on the frequency of applications creating selection pressures against the BLS pathogen, there is a high risk of developing a fungicide-resistant population of M. fijiensis. When resistant populations occur in a region, chemical control will slowly lose its efficacy, leading to a pesticide treadmill phenomena, as described in Chapter I.
Types of fungicides
Fungicides fall in two categories: protectants, or contact fungicides, and systemic fungicides.
Contact fungicides (mancozeb, chlorothalonil) have only a preventive effect and do not penetrate into the banana leaf. They are applied weekly in a systematic manner so that all newly unfurling banana leaves will be protected from new sources of BLS inoculum that come in contact with them. Contact fungicides have a multi-site effect on fungal biology, and therefore strains resistant to these fungicides have never been reported. Their drawback is that the frequency of application is higher, and full coverage of banana leaves is required.
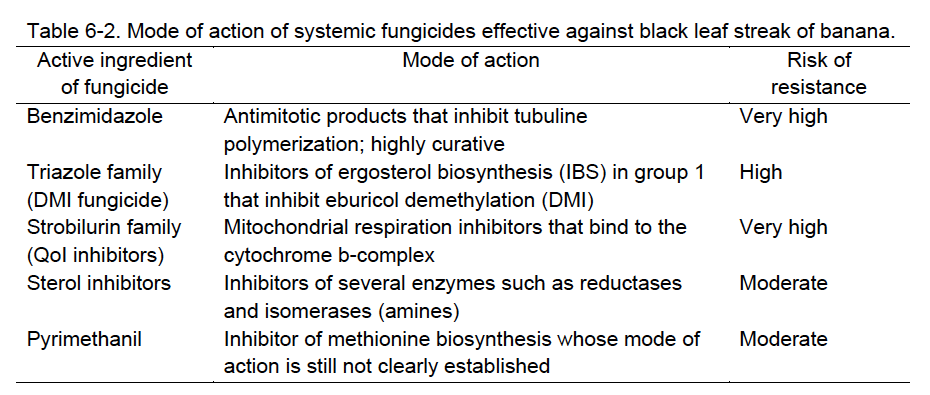
Systemic fungicides penetrate the banana leaf. The systemic properties of these fungicides are variable: some have only a translaminar penetration effect and others have systemic translocation effect throughout the whole plant. Regardless, the mode of action of these fungicides are usually curative. The curative effect is more pronounced on young streaks (stages 1, 2) and lower on older lesions (stages 3, 4), but there is no effect on necrotic stages (stages 5, 6).
Managing fungicide resistance
To avoid the development of a fungicide-resistant M. fijiensis population, farmers can either mix contact fungicides with systemic fungicides in a tank or rotate these two types of fungicides. For example, contact fungicides (e.g., mancozeb or manzate) are commonly rotated with systemic fungicides (e.g., fenbuconazole or tebuconazole). In Hawai‘i, the primary risk of fungicide-resistance development in populations of the BLS pathogen in banana crops is where triazole fungicides (e.g., Enable and Elite) are used. A “block spraying” program rotation was developed by the Hawai‘i banana industry for prudent use of the effective triazole fungicides (Nelson 2009).
For a more efficient fungicide-spraying program,
- Use contact fungicides during the dry season and systemic fungicides mainly in the rainy season.
- Always add penetrants or a spreader sticker in the fungicide spray tank.
- Always conduct sanitary leaf removal before spraying fungicide.
- Develop a disease forecast program to determine when spraying is needed. A BLS disease forecast program is described in detail by Ganry et al. (2012). However, this forecast system is challenged by many factors and is difficult to implement consistently.
- Avoid planting banana suckers contaminated with fungicide-resistant M. fijiensis. Nurseries for planting material must be established in areas where resistant strains have not been found.
- Monitor fungicide resistance of BLS fungal population routinely in your field if possible.
- Special attention should be paid to the management of fungicide resistance that might develop following the repetitive use of curative fungicides.
Monitoring fungicide resistance
The basic methodology relies on the comparison of the sensitivity to different fungicides in fungal populations (50–100 spores) sampled in commercial farms that use these fungicides versus fungal populations sampled in untreated locations. The monitoring of sensitivity is based on germination tests: the germination of spores grown on agar media to which different concentrations of fungicides have been added is compared with the germination of spores grown on agar to which no fungicides have been added (de Lapeyre de Bellaire et al., 2010).
Such an approach was adopted for BLS management when the disease appeared in Gabon on plantains for domestic markets, in Cameroon’s banana-export industry, and on the Ivory Coast and in Ecuador on banana and plantain (Ganry et al. 2012). Through a collaborative area-wide BLS-management program, BLS in these countries has been effectively controlled with an average of 10 to 15 fungicide treatments per year, as compared to 30 to 60 fungicide applications in some Latin American countries where strains resistant to curative (systemic) fungicides had emerged (Carlier et al. 2000, Romero & Sutton 1997). When resistance to systemic fungicides emerges, non-curative contact fungicides are often applied more frequently and at higher dosages (de Lapeyre de Bellaire et al. 2009).
Challenge of Disease Forecasting for BLS Disease
A forecast program that can predict optimal times to apply fungicide for BLS management is based on the earliest visible detection of infection on a banana plant, streak/spot density on cigar leaf (categorized into five stages), and the stage of BLS symptoms (6 stages). However, Ganry et al. (2012) discussed the challenges of this forecast program for fungicide application insofar as it relies on a number of factors:
Little to no resistance to the available fungicides
Availability of systemic fungicides with different modes of action
Logistic capacity to apply fungicides when necessary without delay
Strong area-wide cooperation of farmers and residents in the neighborhood
Access to aircraft for aerial fungicide application.
Not all of the programs are successful in avoiding the development of resistant strains of M. fijiensis. The logistics of flying aircraft for aerial fungicide applications are especially challenging in islands where residential areas are in close proximity to banana farms. Backpack mist blowers provide inferior coverage and control of BLS compared with tractor-drawn mist blowers (Nelson 2009). Calibration of spray coverage is a key component in pesticide efficacy, especially for backpack blowers. For instructions on how to calibrate a backpack mist blower, please refer to “Motorized Back-Pack Mist Sprayer: Sprayer Calibration Using the 1/128th Method” (Uyeda et al., 2013).
References
Beveraggi, A., X. Mourichon, and G. Salle. (1995). Comparative study of the first stages of infection in sensitive and resistant banana plants with Cercospora fijiensis (Mycosphaerella fijiensis), responsible for Black Leaf Streak Disease, Can. J. Bot. 73: 1328–1337.
Carlier, J., E. Fouré, F. Gauhl, D.R. Jones, X. Lepoivre, Mourichon, C. Pasberg-Gauhl, and R.A. Romero. 2000. Fungal diseases of the foliage – Sigatoka Leaf Spots, in “Diseases of banana,abaca and Ensete,” D.R. Jones, Cabi Publishing, U.K., 37–91.
de Lapeyre de Bellaire, L., J. Essoh Ngando, C. Abadie, C. Chabrier, R. Blanco, T. Lescot, J. Carlier, and F. Côte. 2009. Is chemical control of Mycosphaerella foliar diseases of bananas sustainable? Acta Hortic., 828: 161–170.
Ganry, J., E. Fouré, L. de Lapeyre de Bellaire, and T. Lescot. 2012. An integrated approach to control the black leaf streak disease (BLSD) of bananas, while reducing fungicide use and environmental impact, fungicides for plant and animal diseases. D. Dhanasekaran (ed.) InTech, Rijeka, Croatia. (http://www.intechopen.com/books /fungicides-for-plant-and-animaldiseases/an-integrated-approach-to-control-the-black-leaf-streak-disease-blsd-of-bananas-while-reducingfungi).
Kawate, M. 2006. Banana pesticide update. Proceedings of the 37th Annual Hawaii Banana Industry Association Conference. UH-CTAHR Cooperative Extension Service. www2.hawaii.edu/~snelson/HBIA/Banana_Pesticide_Update_2006.pdf
Nelson, S.C., and F. Javier. 2007. Trials of FHIA banana varieties for resistance to black leaf streak in Pohnpei FSM. Proceedings of the 38th Annual Hawaii Banana Industry Association Conference. www.ctahr.hawaii.edu/nelsons/HBIA2007 /Nelson2007.pdf
Romero, R.A., Sutton, T.B. (1997). Sensitivity of Mycosphaerella fijiensis, causal agent of black Sigatoka of banana, to propiconazole. Phytopathology, Vol.87, pp. 96-100.
Uyeda, J., J. Sugano, S. Fukuda, M. Kawate, R. Shimabuku, and
K.-H. Wang. 2013. Sprayer calibration using the 1/128
th method for handheld spray gun systems. CTAHR Cooperative Extension Service PRRE-7.
www.ctahr.hawaii.edu/oc/freepubs/pdf/PRRE-7.pdf