Banana Pest and Disease Management in the Tropical Pacific: A guidebook for banana growers
Chapter V: IPM Strategies against Plant-Parasitic Nematodes on Banana
Common control measures for banana nematodes practiced by farmers

Banana is a perennial crop, which makes management of nematodes on it more challenging than for annual crops. Although pre-plant treatments such as soil fumigation with Telone II® (1,3-dicloropropene) are very effective in suppressing nematode populations, such treatments are short lived compared to the life of a banana plot. Plant-parasitic nematodes tend to repopulate an area gradually after fumigation. Telone potentially contaminates groundwater, is a restricted-use pesticide, and is also rather costly. Because banana is usually cultivated as a perennial crop, a post-plant treatment is essential for nematode management. In terms of post-plant nematicides, fenamiphos (Nemacur®) is environmentally harsh and hazardous to applicators, thus its Special Local Needs label for Hawai‘i has expired. Although erthoprop (Mocap®) and Myrothecium verrucaria (DiTera®) are also effective post-plant nematicides, they are difficult to handle. In 2003, CTAHR and the Hawaii Banana Industry Association developed an integrated pest management program for banana production in Hawai‘i. This includes selecting banana cultivars resistant to or tolerant of plant-parasitic nematodes. Two banana hybrids from the Fundacion Hondureña de Investigacion Agricola (FHIA) breeding program with resistance to black leaf streak disease were tested for their resistance to plant-parasitic nematodes of banana in American Samoa (Brooks 2004). The FHIA-01 hybrid was relatively resistant to burrowing nematodes, whereas FHIA-25 was relatively resistant to all plant-parasitic nematodes commonly feeding on banana in American Samoa.
Most banana growers in Hawai‘i select banana varieties based on their market demand or their tolerance to key pest organisms. Banana farmers prefer to prop up fruiting plants to prevent toppling (Fig. 5-1), treat banana keiki (suckers) to reduce nematode infection, mulch under the plant, or use fertilizer to increase plant vigor rather than using nematicides. The unintentional planting of keiki already infected with nematodes is the principal means of nematode dispersal to uninfested sites. Obtaining nematode-free planting material is therefore the first line of defense to restrict the spread of nematodes. This chapter will review and discuss multiple strategies to reduce initial nematode infection of banana planting materials.
Clean propagative materials
Banana tissue culture
Preventing the spread of pests and diseases is one of several advantages of using tissue-cultured plantlets. Using known nematode-free banana plantlets for planting material is an important mechanism to ensure that nematodes are not being spread around an infected field or introduced to a new field. Since the majority of banana fields in Hawai‘i are already infested with plant-parasitic nematodes (Wang et al. 2009), micro-propagation from disease-free materials using sterile techniques offers a good way to obtain nematode-free planting materials. A banana tissue-culture facility that provided a reliable source of Banana bunchy top virus-free and nematode-free planting material was set up at the University of Hawai‘i at Mānoa Agriculture Diagnostic and Service Center (http://www.ctahr.hawaii.edu/oc/freepubs/pdf/BIO-8.pdf), though unfortunately, due to lack of funding, the program has recently been terminated. For more details on how to tissue-culture banana, please refer to Chapter VIII of this book.
Hot-water treatment

A hot-water dip has been successfully used to control burrowing nematodes in anthurium and root-knot nematodes in ginger. Although treatment recommendations from researchers in different parts of the world vary from 5 minutes at 50°C to 25 minutes at 55°C, CTAHR researchers recommend disinfesting banana suckers by soaking them for 10 minutes at 50°C. A recent experiment with keiki collected from a farm heavily infested with spiral nematodes, combined with low numbers of burrowing nematodes, showed that this hot water treatment was sufficient to kill all nematodes in roots if the size of the corm ranged from 2 to 6 inches in diameter (Table 5-1). In another study, where burrowing nematodes were abundant and had penetrated into the corm, the hot water treatment did not kill the nematodes inside the corm. However, all of the heat-treated keiki grew well when planted in the field. One drawback of this method is that it requires the use of a hot water tank with heater and temperature-control capability (Fig. 5-2), which might not be feasible for small-scale growers to set up.

Sodium hypochlorite dip
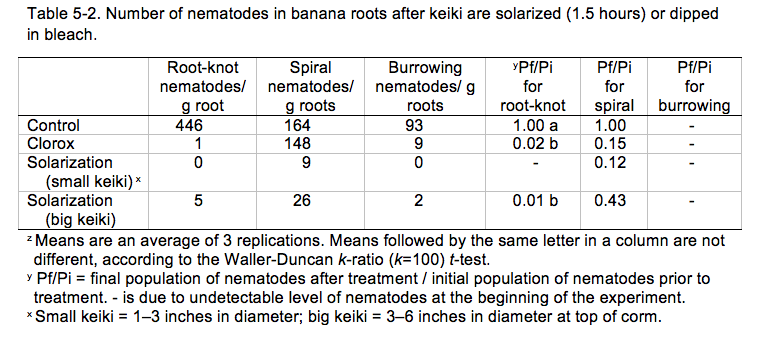
Currently, some banana growers in Hawai‘i concerned with nematode damage treat their suckers by dipping in a diluted household bleach solution—consisting of one part bleach (6.0% sodium hypochlorite or NaOCl, unscented and without other additives) and nine parts water—for 10 minutes prior to planting. Results indicate that such a NaOCl dip reduces 85% of spiral nematodes in the roots as compared to roots that are untreated (Table 5-2). Dipping in bleach may reduce plant vigor, but it is an easily accessible method for most farmers.
Modified solarization

Soil solarization involves heating the soil beneath a clear (transparent) plastic sheet to reach temperatures lethal to soilborne pests. The method has been successfully used against plant-parasitic nematodes and other soilborne pathogens and pests in the top 4 inches (10 cm) of soil, but pests can rebound from a deeper soil layer. A study was conducted using solarization plastic (low-density, 25-µm-thick, UV-stabilized, polyethylene mulch) to suppress nematodes infecting banana keiki collected from the field (Fig. 5-3). Keiki were solarized for different length of times and subsamples of roots were collected before and after solarization. Nematodes were extracted from the roots using the Baerman technique and counted. Although the suppressive effect varies, there was a clear trend that all solarization reduced nematodes in the roots even at 4 hours of exposure time, the shortest time tested in this trial (Table 5-3).
A second experiment was conducted to examine a shorter solarization time (1.5 hours) with different keiki sizes (1–3” vs. 3–6” diameter) and compared to dipping in bleach and untreated control. Results revealed that 1.5 hours of solarization treatment could suppress the plant-parasitic nematodes present significantly as compared to the control for both sizes of keiki (Table 5-2).
Pesticides
As mentioned in the introduction section of this chapter, soil fumigants or nematicides are no longer available for banana production in Hawai‘i except for one biologically based nematicide, Melocon WG® (Certis, Bonita, CA) that contains Paecillomyces lilacinus strain 251 as its active ingredient (HPIRS 2011). Paecilomyces lilacinus is a common soil fungus that has been isolated from many different habitats around the world. It is well known as a facultative egg pathogen of sedentary nematodes and also an important option to control juvenile and adult burrowing nematodes in banana. This nematode-antagonistic fungus may be used in an integrated approach to control plant-parasitic nematodes of banana. Mendoza et al. (2004) demonstrated that nematode activity decreased in the presence of this fungus. For effective control, banana plantlets should be inoculated with P. lilacinus and re-inoculated into the soil at transplanting (Mendoza et al. 2004).
On the other hand, since the most damaging nematodes on banana spend a significant amount of their life cycle inside root tissue, killing nematode-infected plants with bananacide (glyphosate) also kills the endoparasitic stage of the nematodes and thus greatly improves the potential of the successive fallow practice to reduce nematode infestation without nematicides (Chabrier & Quénéhervé 2003). Commercial banana growers in the Caribbean and Africa are recommended to integrate bananacide application as part of their banana IPM program to combat plant-parasitic nematodes. In fact, in Martinique, bananacide injection to kill old and yield-declining banana plants, followed by a fallow period and replanting with tissue-cultured banana plants, has extended banana field longevity from 3–4 to 6–10 years, and in some cases contaminated fields have been totally freed from burrowing nematodes (Chabrier & Quénéhervé 2003).
Cover-cropping
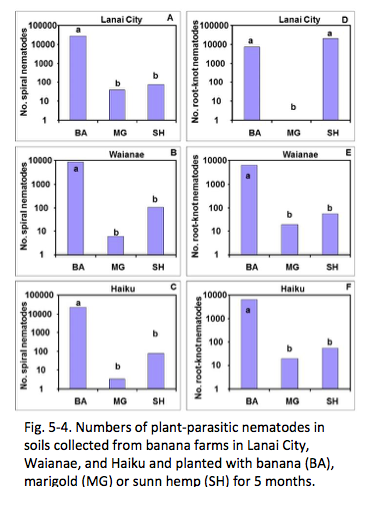
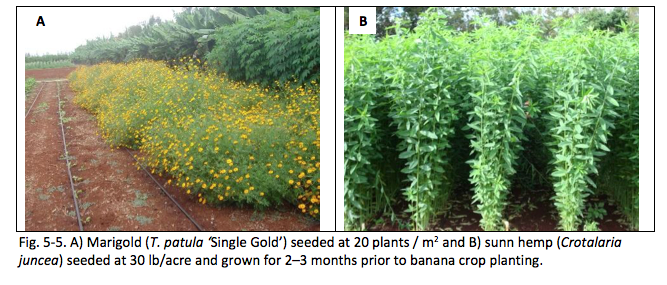
Since banana is a perennial crop, managing nematodes over a longer period of time after planting is critical. Planting low-growing cover crops that release nematode-allelopathic compounds is an ideal option. Allelopathy is a biological phenomenon by which an organism produces one or more biochemicals that negatively affect the growth, survival, and reproduction of other organisms. Example of cover crops with allelopathic effects against plant-parasitic nematodes include sunn hemp (Crotalaria juncea), marigold (Tagetes spp.), rapeseed (Brassica napus, Wang et al. 2001), velvetbean (Mucuna pruriens, Zasada et al. 2006), sorghum-sudangrass (Sorghum bicolor × Sorghum arundinaceum var. Sudanense, Widmer and Abawi 2000). Among these cover crops, marigold stands out as an especially suitable candidate for banana cropping systems due to its low-growing habit and because it does not require soil incorporation to be allelopathic. The allelopathic effect of marigold varies according to marigold and nematode species, cultivar, and soil temperature (Ploeg and Maris 1999). In field trials, Tagetes patula ‘Single Gold’ consistently suppressed a diverse range of plant-parasitic nematodes. Only living marigold root systems exhibit nematicidal properties; incorporation of ‘Single Gold’ residues into the soil does not suppress root-knot nematode (Ploeg 2000).
Planting of T. patula and C. juncea in field soil infested with plant-parasitic nematodes significantly reduced the nematode population densities after 5 months as compared to continuous planting of banana into these soils in a greenhouse experiment (Fig. 5-4). Thus, both of these cover crops can be planted prior to banana crop planting to reduce population densities of plant-parasitic nematodes (Fig. 5-5). Since marigold can be planted as a low-growing living mulch, and because it releases its nematicidal compounds while growing, it is more compatible with the banana crop than sunn hemp. The next logical step is to determine the optimum planting time of marigold as a cover crop after banana planting so as 1) not to compete with banana growth and 2) not to be shaded by banana canopy.
Two field trials indicated that marigold could not survive if transplanted into an 18- or 36-month-old banana field, mostly due to shading. A second experiment was conducted to compare banana and marigold growth if the marigold was transplanted within 6 months of banana planting. Marigold was planted at monthly intervals. At 8 months after transplanting, banana grown in conjunction with marigold seedlings that were planted 4 to 6 months after the banana grew better than the no-marigold control (Fig. 5-6). Marigold grew better when planted 4 to 5 months after banana planting (Fig. 5-7). Therefore, if using marigold as a post-plant cover crop, farmers should aim to plant it at 4 to 5 months after banana planting to maximize marigold growth and minimize competition from banana growth (Fig. 5-8).
Although plantings of marigold under the banana canopy will slowly decline over time as the banana canopy fills in, marigold nonetheless will reduce the population densities of plant-parasitic nematodes infecting the banana over a longer period of time compared to no marigold planting. Data from the Caribbean revealed that longevity of banana in a nematode-infested field is generally 3–4 years (Chabrier & Quénéhervé 2003). Concerned farmers would replant their field every 3–4 years. A similar trend is observed in Hawai‘i: a banana farmer in Lana‘i has observed that their banana plants decline in yield over time, and they generally replant every 5 years to maintain a stable yield (de Jetley, personal communication). We anticipated that efficient management of plant-parasitic nematodes at pre-plant, followed by post-plant management with marigold planting, would prolong the longevity of banana with good yield beyond 5 years in Hawai‘i.
![Fig. 5-6. Banana plant growth differences (final height - initial height) affected by months since marigold transplanting. Means are average of 4 replications. Columns with same letters are not different according to Waller-Duncan k-ratio (k=100) t-test. C=control with no marigold. Fig. 5-7. Marigold growth [(Final height - initial height)/initial height] under banana plants affected by months since marigold transplanting. Means are average of 4 replications. Columns with same letters are not different according to Waller-Duncan k-ratio (k=100) t-test. Fig. 5-6. Banana plant growth differences (final height - initial height) affected by months since marigold transplanting. Means are average of 4 replications. Columns with same letters are not different according to Waller-Duncan k-ratio (k=100) t-test. C=control with no marigold. Fig. 5-7. Marigold growth [(Final height - initial height)/initial height] under banana plants affected by months since marigold transplanting. Means are average of 4 replications. Columns with same letters are not different according to Waller-Duncan k-ratio (k=100) t-test.](https://gms.ctahr.hawaii.edu/gs/handler/getmedia.ashx?moid=30272&dt=3&g=5)
Home Gardener’s Corner

Home gardeners should take advantage of the reduced shading from the banana canopy, as only few plants will be planted in home yards. It is recommended to plant French marigold as a ground cover surrounding the perimeter of a banana mat. Gardeners should keep up with a good banana management practice and maintain a banana mat with no more than 3 plants per mat. This will ensure better banana bunch yield and also allow marigold to have access to sunlight over a longer period of time and thus continue to suppress the population densities of nematodes.
References
Brooks, F.E. 2004. Plant-parasitic nematodes of banana in American Samoa. Nematropica 34:65–72.
Chabrier, C. and Quénéhervé, P. 2003. Control of the burrowing nematode (Radopholus similis Cobb) on banana: Impact of the banana field destruction method on the efficiency of the following fallow. Crop Protection 22:121–127.
Hooks, C.R.R., Chandara, K., Fallon, D., K.-H. Wang, and R. Manandhar. 2007. The impact of sunn hemp cover cropping on belowground organisms and nutrient status associated with a cucumber planting. Cooperative Extension Service SCM-21.
HPIRS, 2011. Pesticides for use on banana in Hawaii. http://pesticides.hawaii.edu/hipmrinn/hipmrinn.htmlhttp://pesticides.hawaii.edu/hipmrinn/hipmrinn.html
Mendoza, A., R.A. Sikora, and S. Kiewnick. 2004. Efficacy of Paecilomyces lilacinus strain 251 for the control of Radopholus similis in banana, Commun. Agric. Appl. Biol. Sci, 69: 365–372.
Ploeg, A.T. 2000. Effects of amending soil with Tagetes patula cv. Single gold on Meloidogyne incognita infestation of tomato. Nematol. 2: 489–493.
Ploeg, A.T. and P.C. Maris. 1999. Effect of temperature on suppression of Meloidogyne incognita by Tagetes cultivars. J. Nematol. 31: 709–714.
Wang. K.-H. and C.R.R. Hooks. 2009. Survey of nematodes on banana in Hawaii and methods used for their control. CTAHR Cooperative Extension Service PD-69. 7pp. http://www.ctahr.hawaii.edu/oc/freepubs/pdf/PD-69.pdf
Wang, K.-H., C.R.R. Hooks, and A. Ploeg. 2007. Protecting crops from nematode pests: using marigold as an alternative to chemical nematicides. Cooperative Extension Service, CTAHR, PD-35.
Wang, K.-H., B.S. Sipes, and D.P. Schmitt. 2001. Suppression of Rotylenchulus reniformis by Crotalaria juncea, Brassica napus, and Target erecta. Nematropica 31: 237–251.
Widmer, T.L. and G.S. Abawi. 2000. Mechanism of suppression of Meloidogyne hapla and its damage by a green manure of sudangrass. Plant Dis. 84: 562–568.
Zasada, I.A., W. Klassen, S.L.F. Meyer, M. Codallo, and A.A. Abdul-Baki. 2006. Velvetbean (Mucuna pruriens) extracts: impact on Meloidogyne incognita survival and on Lycopersicon esculentum and Lactuca sativa germination and growth. Pest Management Sci. 62:1122–1127.