Banana Pest and Disease Management in the Tropical Pacific: A guidebook for banana growers
Chapter IV: IPM Strategies against BBTV
BBTV
Banana bunchy top virus (BBTV) is a circulative, persistent, but non-propagative virus (as described in Chapter II) in the family Nanoviridae, genus Babuvirus, BBTV, transmitted specifically by the banana aphid, Pentalonia nigronervosa Coquerel (Hemiptera, Aphididae). This means that the virus, once acquired by the aphid vector, will persist in the vector throughout the life of the aphid, but the virus will not reproduce in the aphid. Instead, the virus will reproduce to a higher titer concentration in the banana plant. Farmers should be aware that once a banana plant is infected, the virus will be translocated through the phloem tissue of the plant and travel throughout the entire plant mat. Since there is no therapeutic remedy for BBTV once a plant is infected, IPM strategies against BBTV should focus on stopping the spread of the disease.
Current tactics recommended for mitigating BBTV spread in Hawai‘i include: 1) early detection of virus-infected plants, 2) timely destruction of virus-infected plants with a bananacide (herbicide), 3) insecticidal treatment on virus-infected plants, 4) managing banana aphid populations on banana, 5) replanting with virus-free banana plants (Hooks et al. 2014); 6) managing banana aphids on alternative hosts, 7) controlling ants, and 8) growing tolerant varieties (Nelson et al. 2006). Some of these recommendations are being verified and improved by researchers at the University of Hawai‘i and are summarized in this chapter.
Early detection of virus-infected plants
Early symptom expression of BBTV is described in Chapter II Fig. 2-1. Unfortunately, a banana infected by BBTV will go through a latent period before showing symptoms. A report from Australia estimated that the incubation period for BBTV is 125 days (Allen 1978), but in Hawai‘i, the range is 20 to 85 days after aphid inoculation, with most plants displaying symptoms by 50 days after inoculation (2008; 2009). While early detection of BBTV infection through molecular diagnostics is available, it will not be feasible for farmers with acreages of banana plants to submit every plant for testing. Thus, an IPM program for BBTV management should go beyond early detection and removal of BBTV symptomatic plants.
Destruction of BBTV-infected plants with a bananacide
Once a banana plant is diagnosed with BBTV, it should be destroyed immediately to mitigate the spread of the virus. BBTV-infected plants can be destroyed by injecting them with bananacide (an herbicide registered for killing banana plants). A list of bananacides registered for use in Hawai‘i can be found at the Hawai‘i Pesticide Information Retrieval System (HPIRS) (http://pesticides.hawaii.edu/hipmrinn/hipmrinn.html). Previously, it has been suggested that if a symptomatic plant is found, growers should consider rogueing healthy neighboring plants (Allen 1978; Magnaye and Valmayor 1995). In a study where Hooks and Wang closely monitored and destroyed BBTV-infected plants weekly and recorded BBTV movement in banana plantings for 3 years, they found that banana plants neighboring BBTV infected plants remained healthy throughout the 3-year study (Hooks and Wang unpublished data). The practice of destroying healthy plant mats next to BBTV-infected plant mats has most likely resulted in unwarranted labor and loss of income. A better strategy is to scout for BBTV-symptomatic plants regularly, promote timely destruction of infected plants, and replant with BBTV-free planting materials such as tissue-cultured plants as illustrated here.

Insecticidal treatment
To prevent the spread of BBTV, managing the population densities of banana aphids is key to an IPM program against BBTV. A list of insecticides registered for use on banana in Hawai‘i can be found at the Hawai‘i Pesticide Information Retrieval System (HPIRS) (http://pesticides.hawaii.edu/hipmrinn/hipmrinn.html). Among the insecticides listed in HPIRS that are effective against banana aphids, imidacloprid is a systemic insecticide that acts as an insect neurotoxin. Users must follow the label to apply the insecticide, including method of application, number of permitted applications per year, application rate, personal protective equipment (PPE) required, preharvest (PEI) and restricted-entry intervals (REI), etc. Since this insecticide is systemic, it is not necessary to spray the entire plant to reach effective control of the pest. However, ensuring the permeability of the insecticide into the banana plants will increase the effectiveness of the insecticide. This can be done by adding compatible spreader stickers and removing leaf residue that will block the spray contact (Fig. 4-6). Newly registered systemic insecticides for banana are in development, and growers are encouraged to develop an insecticide-rotation program to avoid the buildup of pesticide-resistant aphid populations. An insecticide rotation program should include insecticides with different modes of action that can usually be identified by Group number on the label (Fig. 4-7). Pesticides with different modes of action kill insects differently and thus are less likely to create a pest population resistant to pesticides. Other insecticides listed on HPIRS to combat banana aphids include neem products, fine-spray oil, potassium salts of fatty acids, etc. These are broad-spectrum insecticides that can kill aphids through contact, which can be integrated into a pesticide rotation program.

Insecticides can be used to manage aphid populations when BBTV is detected and at the time of rogueing. This strategy of spraying only BBTV-infected plants may be extended to neighboring banana mats in the likelihood that a neighboring plant is infected. This practice will reduce the risk of secondary virus spread without the wasteful destruction of neighboring healthy banana plants, and thus help improve the efficiency of rogueing with bananacide (Hooks et al. 2009). Compared to preemptive sprays of insecticide directed at the entire banana plantation, this approach is more cost effective.
The importance of BBTV-infected but asymptomatic banana plants to the spread of BBTV is unknown. Thus, managing banana aphid populations may be important even if disease incidence is unknown. It is understandable that farmers who have suffered from heavy infestation of BBTV may wish to spray preemptively as crop insurance. However, a versatile IPM program emphasizes pest biology, economic thresholds, environmental factors, and host plant resistance if feasible. It is important to prevent the development of a pesticide-resistant pest population and avoid the over-application of insecticides.
A sampling plan and economic threshold for banana aphids on banana was developed by researchers at the University of Hawai‘i (Robson et al. 2006, 2007). Previously, growers and Extension personnel inspected the most recently unfurled leaf (i.e., cigar leaf) of a banana plant to check for the presence of banana aphids. However, a study conducted by Robson et al. (2006) found that inspecting only cigar leaves for banana aphids led to 59% false negatives. However, they found that if they sampled the bottom two leaves, only 6% came back as false negatives for banana aphids. Thus, if farmers sample the bottom two leaves for banana aphids, they can determine whether they should treat with insecticides based on the binomial sequential sampling plan shown in Fig 4-8.
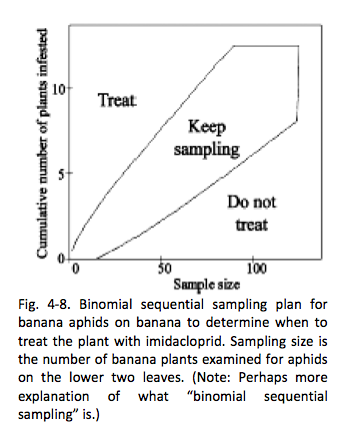
However, there is an important addition to this recommendation. Robson et al. (2007) later reported that while imidacloprid foliar application on bananas resulted in effective control of aphids on old and new leaves that come in direct contact with the insecticide over a 4-week testing period, the pesticide does not become thoroughly systemic within the plant. New leaves that emerge after spraying will not be completely protected from aphids because imidacloprid is not truly a systemic insecticide, but rather a membrane-permeable insecticide. Thus, regular scouting for aphids on newly unfolded leaves should still be implemented when applying imidacloprid specifically.
In terms of spray coverage, conservative farmers tend to think that total coverage with insecticide is needed to control an aphid population. A study on the distribution of banana aphids within a banana mat through a survey conducted on 25 banana farms in Hawai‘i (20–45 banana mats per farm) showed that only 7.6% of the plants examined had aphids distributed higher than 2.5 m (8.2 ft) from the base of the plant (Hooks et al. 2011). Depending on plant biomass and planting densities, full spray coverage of banana crops could reach > 200 gal per acre. The drawback of spraying with such a high spray volume is 1) potential dilution of allowable insecticide application rate and 2) waste of pesticide spray in areas where aphids are not found. Population densities of banana aphids are higher in plants < 1.5 m tall than in those > 1.5 m tall (Hooks 2011). Understanding the distribution of banana aphids on banana plants can provide farmers a better pest management strategy.
The strategy of injecting bananacide and spraying insecticide only on infected plants could 1) significantly reduce the cost of pesticide applications, 2) eliminate preemptive insecticidal spraying throughout the entire orchard, 3) reduce the potential for the banana aphid to develop insecticidal resistance, and 4) avoid unwarranted destruction of healthy plants.
Replanting with virus-free plants
In a BBTV-infested site in Hawai‘i, if 50 newly transplanted symptomless suckers are examined, 100% of them tend to have aphids, 92% have winged aphids, and 20% of those aphids are infected with BBTV (Hooks personal communication). The probability that newly planted, apparently healthy suckers are infected with BBTV is high. If visible symptoms of infection only become obvious at 20 to 85 days after aphid inoculation (Hooks et al. 2008 and 2009), planting virus-free tissue-cultured plants is an important aspect of reviving a BBTV-infested site. A banana tissue-culture program was established 2007 to early 2014 at the University of Hawai‘i Seed Lab to distribute BBTV-free tissue-cultured banana. Lack of financial support resulted in the closing of this service, but the program demonstrated to farmers the value of establishing virus-free planting materials. Farmers in need of tissue-cultured banana plantlets are learning to work with other tissue-culture laboratories that provide the services. The Hawai‘i Agriculture Research Center (HARC) provides micro-propagation services upon special order. If resources are available, some banana farmers can establish their own tissue-culture laboratories to mass-produce banana varieties of their interest. Please refer to Chapter VIII on how to initiate a banana tissue-culture program.
Managing banana aphids on alternative hosts?
BBTV is usually only transmitted by banana aphids (P. nigronervosa). A close relative of banana aphid, Pentalonia caladii, has become a concern as another BBTV vector, but the concern may have been blown out of proportion. Both P. nigronervosa and P. caladii are believed to reproduce exclusively asexually in most subtropical and tropical regions, including the Pacific. In nature, they occupy different hosts. Pentalonia nigronervosa primarily colonizes banana (Musa spp.) and taro (Colocasia esculenta) plants, whereas P. caladii chiefly colonizes ginger (Zingiber officinale, Alpinia purpurata, Hedychium coronarium), heliconia (Heliconia spp.), and taro plants (Foottit et al. 2010). However, some sexually reproductive forms of Pentalonia aphids have been reported in northeast India and Nepal (Blackman and Eastop 2000), and both aphid species have the potential to exploit common hosts (Bhadra and Agarwala 2010). In addition, researchers at the University of Hawai‘i showed for the first time that P. caladii is a competent vector of BBTV and is capable of acquiring the virus from infected banana plants and transmitting it to other banana plants (Watanabe et al. 2014). Ginger, heliconia, and taro plants often grow in close proximity to banana fields in Hawai‘i, which raises a concern about the need to manage Pentalonia aphids. However, Hu et al. (1996) conducted transmission experiments showing that taro and ginger plants cannot serve as hosts for BBTV, and P. caladii displays a strong host plant preference (Foottit et al. 2010). Therefore, the belief that managing BBTV also requires managing aphids on ginger, heliconia, and taro is a myth.
Is ant control necessary?
Previously it was recommended that controlling ants was important to lower aphid populations. However, Hooks et al. (2011) reported that the probability of observing ants on banana plants without aphids was 41, 49, and 63% on suckers < 1.5 m tall, suckers 1.5–2.5 m tall, and the mother plant (> 2.5 m tall), respectively. This would mean that 41–63% of the time farmers would be trying to control ants on banana that are not associated with aphids. Thus, managing ant populations is not necessary as long as the aphid population is not high.
Grow BBTV-tolerant varieties
Hawai‘i stakeholders commonly believe that the variety ‘Dwarf Brazilian’ or ‘Santa Catarina’ (locally known as “dwarf apple”) is less susceptible to BBTV than the ‘Williams’ banana. Hooks et al. (2009) reported that banana aphids transmitted BBTV to both cultivars at a similar rate (>90% for both cultivars) in a laboratory experiment. Field results, however, showed a lower percentage of dwarf apple (39%) plants infected with BBTV compared to ‘Williams’ (79%), despite the similar incubation period (time required from BBTV infection to symptom expression). It has been speculated that the waxy surface of the dwarf apple banana plant caused the banana aphids to fall off more easily. This made a difference in the field infection rate of BBTV between the two varieties (Hooks et al. 2009). Screening of banana cultivars for resistance to BBTV is currently underway at the University of Hawai‘i (Sachter-Smith and Manshart, personal communication). A preliminary comparison of banana cultivar susceptibility to BBTV infection at 6 months after inoculation is shown in Fig 4-9.

Home Gardener’s Corner

It is equally important for home gardeners to rogue out, or find and destroy, BBTV-infected banana as it is for the commercial banana growers. This will have a big impact on the effort to safeguard the banana industry in Hawai‘i.
Web Resources
Nelson, S., L. Richardson, et al. 2006. BBTV in Hawaii. University of Hawai‘i at Manoa. https://www.ctahr.hawaii.edu/bbtd/video.asp
References
Bhadra, P. and B.K. Agarwala. 2010, A comparison of fitness characters of two host plant-based congeneric species of the banana aphid, Pentalonia nigronervosa and P. caladii. J. Insect Sci. 10.
Foottit, R.G., H.E.L. Maw, K.S. Pike, and R.H. Miller. 2010. The identity of Pentalonia nigronervosa Coquerel and P. caladii van der Goot (Hemiptera: Aphididae) based on molecular and morphometric analysis. Zootaxa 25–38.
Hooks, C.R.R., K.-H. Wang, N.C. Pradhan, R. Manandhar, A. Varsino, and M.G. Wright. 2011. Population distribution and density of Pentalonia nigronervosa Coquerel (Hemiptera: Aphididae) within banana mats: Influence of plant age and height on sampling and management. J. Econ. Entomol. 104: 947–955.
Hu, J.S., M. Wang, D. Sether, W. Xie, K.W. Leonhardt. 1996. Use of polymerase chain reaction (PCR) to study transmission of Banana bunchy top virus by the banana aphid (Pentalonia nigronervosa). Ann. Appl. Biol. 128: 55–64.
Robson, J.D., M.G. Wright, and R.P.P. Almeida. 2006. Within-plant distribution and binomial sampling of Pentalonia nigronervosa (Hemiptera: Aphididae) on banana. J. Econ. Entomol. 99: 2185–2190.
Robson, J.D., M.G. Wright, and R.P.P. Almeida. 2007. Effect of imidacloprid foliar treatment and banana leaf age on Pentalonia nigronervosa (Hemiptera: Aphididae) survival. New Zealand J. Crop and Hort. Sci. 35: 425–422.
Watanabe, S., A.M. Greenwell, and A. Bressan. 2013. Localization, concentration, and transmission efficiency of banana bunchy top virus in four asexual lineages of Pentalonia aphids. Viruses 5: 758–775 (doi:10.3390/v5020758).